what happens to the auditory ossicles in otitis media
Otitis Media With Effusion: Comparative Effectiveness of Treatments
Research Protocol ARCHIVED Mar 20, 2012
On July 30, 2012, amendments were made to this protocol. To view these amendments, please see the section titled "Summary of Protocol Amendments."
Groundwork and Objectives for the Systematic Review
Otitis media with effusion (OME) is defined as a collection of fluid in the middle ear without signs or symptoms of ear infection.1 It typically arises when the Eustachian tubes are not functioning normally. When this happens, pressure changes occur in the centre ear and fluid can accrue.
OME is 1 of the nearly usually occurring childhood illnesses in the Us with more 2.ii million diagnosed cases each yr at an estimated annual toll of 4 billion dollars.2 As many every bit xc percent of children (eighty% of private ears) will have at least one episode of OME by age x, with the majority of cases occurring between the ages of 6 months and iv years.2,three Many episodes of OME resolve spontaneously within 3 months, but 30 to xl percent of children accept recurrent episodes and 5 to 10 percent of cases last more than than one year.1,4,5 Additionally, some subpopulations of children are disproportionately affected by OME. Those with cleft palate, Down syndrome, and other craniofacial anomalies are at high hazard for anatomic causes of OME in addition to worsened function of the Eustachian tube.6 Individuals of American Indian, Alaskan, and Asian backgrounds are believed to exist at greater risk,7 as are children with adenoid hyperplasia. In add-on, children with existing hearing loss volition exist affected more dramatically past the secondary conductive hearing loss that occurs with OME.
In that location are several predisposing ecology factors that are associated with an increased risk of developing OME.3 These include exposure to secondhand fume, attending child care, and having environmentally induced allergies.
Although rare, OME likewise occurs in adults, usually developing afterward a severe upper respiratory infection such as sinusitis, severe allergies, or rapid alter in air pressure (barotrauma) after a aeroplane flight or a scuba dive. The incidence of prolonged OME in adults is not known, but information technology is much less mutual than in children.8
OME can be associated with discomfort and a feeling of fullness in the ear. Patients with OME are also prone to episodes of acute otitis media (AOM). Temporary hearing loss is common amongst OME patients. This hearing loss is frequently mild (i.e., worsened or with hearing threshold elevated by about 10 dB), but in some cases moderate or severe hearing loss can occur.9 Considering hearing loss in young children may delay or permanently change their communication skills and may pb to behavioral and educational difficulties,ten in that location has been concern virtually the possible role of OME on these outcomes. Additionally, those with chronic Eustachian tube dysfunction and OME are at run a risk for structural impairment of the tympanic membrane.11
Taking a conscientious history is important to identify chance factors for developing OME. For example, it tin be helpful to arm-twist a history of recent upper respiratory infection, allergy, subjective hearing loss or imbalance, speech and language filibuster, and a history of crevice palate or Downward syndrome.
Diagnostically, OME must be first identified and then distinguished from AOM. OME is diagnosed with the presence of fluid behind the tympanic membrane, without acute onset or signs of inflammation or infection. AOM on the other manus, while information technology may include Eustachian tube dysfunction and middle ear fluid, information technology must include signs of acute inflammation or infection. Another distinguishing feature between AOM and OME is the advent of the tympanic membrane, which bulges with AOM and is typically retracted or neutral with OME. With OME, the tympanic membrane is often cloudy with impaired mobility. Additionally, an air-fluid level or bubble(southward) may exist visible in the eye ear.10 The employ of pneumatic otoscopy to demonstrate decreased mobility of the tympanic membrane is considered an important primary diagnostic method.1 Other factors that help confirm the diagnosis include a flat tympanogram (Type B tympanogram) and a conductive hearing loss on pure-tone audiometry. Hearing is generally measured across the speech range, and for young children normal hearing is considered to be no worse than fifteen dB (which is the measure out of loudness needed to respond to a sound).12 In contrast, the average hearing levels for ears with OME often measure at 25 dB, with nearly 20 percent exceeding 35 dB.i Though usually not necessary to brand a diagnosis, middle ear effusion tin can exist demonstrated on imaging studies, such as computed tomography (CT) of the temporal bone.
Tympanocentesis (use of a needle to puncture the tympanic membrane to allow for fluid drainage, aeration), ordinarily performed at the fourth dimension of myringotomy with or without tympanostomy tube placement, remains the gold standard for diagnosing OME. While AOM may also present with fluid behind the tympanic membrane, it is defined as also including an acute onset of signs and symptoms of centre-ear inflammation.x
Given the natural history of OME, especially in relation to the high case of spontaneous resolution, clinical decisions are complicated, and despite contempo practice guidelines and systematic reviews,vi,10,xiii-nineteen the comparative benefits and harms of treatments and treatment strategies for OME are uncertain.
Table ane lists the various surgical and non-surgical treatments and overall strategies for treating OME. During topic refinement, nosotros looked at each treatment in terms of uncertainty within the published literature (including gaps in the bear witness), clinical importance, patient important outcomes, and relevance to the U.Due south. population.
| Surgical Interventions |
|---|
| Myringotomy |
| Tympanostomy tubes |
| Adenoidectomy with or without myringotomy |
| Pharmacological Interventions |
| Antibiotics and antimicrobials |
| Nasal steroids |
| Oral steroids |
| Antihistamine and decongestants |
| Nonsurgical and Nonpharmacological Interventions |
| Autoinflation of the Eustachian tube |
| Complementary and alternative therapies |
| Hearing aids |
| Treatment Strategies |
| Watchful waiting/delayed treatment/firsthand treatment |
| Variations in surgical technique and procedures |
Treatments That Will Not Be Addressed in This Review: Rationale for Exclusion
The use of antihistamines and decongestants for the handling of OME has been extensively studied in primary randomized controlled trials (RCTs) and summarized in recent systematic reviews19, twenty and clinical do guidelines.6,10 A Cochrane review of OME for use in children identified 16 RCTs that included over ane,800 subjects.nineteen The effects on multiple short- and long-term outcomes repeatedly demonstrated no do good for use of these medications over placebo for treating OME. Additionally, the reviewed studies constitute evidence of increased side furnishings and harms with the employ of these medications. High-quality evidence unequivocally demonstrates that antihistamines and decongestants offer no improvement over placebo, and there is no reason to believe that this will alter with future advances in the medication class or causes of OME. We accept, therefore, decided to exclude antihistamines and decongestants from the current review as a handling that is definitively not effective and likely harmful.
Antibiotics currently are not commonly used in the The states to treat OME and are not recommended in electric current U.S. guidelines.x There is some conflicting testify regarding the effectiveness and utility of antibiotics for the treatment of OME.half dozen,ten,20 An upcoming Cochrane Collaboration review on the use of antibiotics for the treatment of OME in children was started in 2011 and is well underway.21 We will not indistinguishable their efforts and have excluded antibiotics from the current comparative review.
Hearing aids are not used as a treatment option for OME in the Usa and, according to a 2008 National Collaborating Eye for Women's and Children's Health (Squeamish) guideline,6 at that place are no high-quality comparative studies evaluating the effectiveness of hearing aids to other interventions for treating OME. Furthermore, we did not detect any comparative studies on hearing aids during topic refinement, and our Key Informants did not consider hearing aids of clinical relevance in the context of OME. Hearing aids, therefore, will not be included in the electric current review.
Treatments and Treatment Strategies That Will Be Addressed in This Review: Rationale for inclusion
The benefits and harms of oral and topical nasal steroids in treating hearing loss in children with OME was the focus of a recent Cochrane Collaboration review (2006).xviii The review was limited to RCTs identified through May 2006, of either steroid use alone or in combination with another amanuensis such every bit antibiotics, and included special populations of children of interest to our electric current review. Oral steroids alone or in combination with antibiotics had a positive issue on 2-calendar week disease outcomes just not on longer term outcomes. Topical nasal steroids were also plant to accept a minimal result in treating OME in two included studies. Current guidelines developed past both the U.k.'s National Collaborating Centre for Women's and Children'due south Health (2008)half dozen and the American University of Pediatrics (2004)10 recommend against using oral or topical nasal steroids in treating children with OME. For the purposes of identifying the relevant literature for this review, nosotros assume that the Cochrane Collaboration review identified all relevant RCTs as of the time of their review. We volition search the published literature for studies on treating OME with either oral or topical nasal steroids in children and will only consider re-reviewing this intervention if we find new evidence, RCTs published in May 2005 or later (1 year before the last literature search in the Cochrane review), or observational studies from whatever time. In consultation with our Technical Expert Panel (TEP), nosotros conclude that information technology would be useful to integrate newly identified studies with those previously identified through the Cochrane Collaboration review, because the newly integrated studies may result in conclusions different from those of the earlier review.18 Nosotros will conduct a completely new search to identify studies pertaining to adults, because nosotros did not find an existing review focusing on this population.
Adenoidectomy as a handling for OME in children has also been recently reviewed in a 2010 Cochrane Collaboration systematic review.16 The review included seven RCTs comparing adenoidectomy (with or without tympanostomy tubes) and nonsurgical management or tympanostomy tubes only; studies of children up to xviii years of age; followup of six months or longer; and not limited to otherwise healthy children. Our search strategy volition be to assume that this Cochrane 2010 review16 identified all relevant studies relating to both special populations and otherwise healthy children in the literature at the time of the review. Nosotros volition search for RCTs published in March 2008 frontwards (1 twelvemonth before the terminate of the before search) and observational studies from any fourth dimension. We are non aware of any reviews of adenoidectomy in adults with OME; therefore, we will search the literature to locate whatsoever relevant studies.
Though not in widespread utilize, the technique of autoinflation has been used as a treatment for OME. The goal of autoinflation is to utilise either a Valsalva maneuver or external device to equalize middle ear and oropharyngeal pressure, essentially transiently opening the Eustachian tube. A 2006 Cochrane Collaboration study included six RCTs examining the use of autoinflation versus no treatment for hearing loss associated with OME.17 Studies included children, adults, and special populations and concluded that the evidence for the use of autoinflation in the brusk term was favorable; however, given the small number of studies and lack of long-term followup, the long-term furnishings could not exist determined. We will begin with the studies identified in the 2006 Cochrane review and will search for new RCTs published since August 2005 (1 twelvemonth before the concluding search conducted for the 2006 Cochrane review) and observational studies published at any time. We will newly synthesize the literature related to this intervention if we determine that there are sufficient data to examine the efficacy of autoinflation in subpopulations and/or the comparative effectiveness of autoinflation relative to other handling options.
In that location is a literature on complementary and culling medicine (CAM) interventions to treat OME. The volume Bear witness-Based Otitis Media 22 lists treatments and supportive studies for at least ii CAM approaches including concrete manipulation and restricted diets. Based on the recommendations of our TEP, in the electric current review we will only include RCTs of CAM interventions.
Although the most recent guidelines for treating OME practise non recommend the use of myringotomy solitary,x more recent literature suggests that laser-assisted myringotomy may be a useful alternative to myringotomy plus tympanostomy tubes. These recent studies suggest that information technology may provide a handling with fewer complications for selected subgroups of children and adults.23-26 Considering there have been no systematic reviews that accept addressed the effectiveness of myringotomy alone, we will search for relevant RCTs and observational studies examining myringotomy alone as a treatment strategy for OME in otherwise healthy children, special populations of children, and adults.
The harms and benefits of tympanostomy tubes for managing OME in children have been addressed by two recent systematic reviews.thirteen, xv A 2010 Cochrane review of 10 RCTs,fifteen limited to otherwise healthy children, ended that tubes are beneficial for the outcome of hearing in the short term, simply the size of the do good diminishes after 6 to 9 months with no differences seen at 12 and xviii months. With express information bachelor, no furnishings were detected on language or speech evolution or cerebral or quality-of-life outcomes. At least v of the included studies considered long-term outcomes, post-obit children eight years postsurgery. A 2011 systematic review, deputed by the Swedish Quango on Technology Cess in Health Intendance,13 looked at tympanostomy tubes as a treatment for OME, non excluding special populations of children. Based on eight RCTs that followed children for as long equally ten years, the review concluded that there was strong evidence that tympanostomy tubes improve hearing and quality of life in the short term (up to ix months). Comparators included no handling, watchful waiting, and other established treatments. For this review, we will brainstorm with the RCTs that were identified in both of these earlier systematic reviews and will search for new RCTs from April 2006 forward (1 yr earlier the terminal search that included all children) and observational studies from any time. In add-on, we will search for relevant studies in the adult population.
A growing body of literature examines variations in tympanostomy tube-related surgical techniques and procedures for treating OME. The 2011 Swedish systematic review described higher up13 considered various characteristics of tube pattern and surgical procedures and concluded that at that place is equally nevertheless insufficient show to determine if the blueprint of the tube material or variations of surgical procedure has an impact on function. We will search from April 2006 forrard (i twelvemonth earlier the last search of the Swedish systematic review) for relevant studies comparing tympanostomy tube materials, designs, and surgical procedures.
Watchful waiting, or active observation equally it has more recently been chosen, is the process of regular review and followup of the child, including assessments of hearing, development, and educational progress. We will examine this as a handling strategy, distinct from "no treatment." Watchful waiting has not been the focus of a systematic review, although it has been a comparator in RCTs included in systematic reviews focusing on other interventions. Current clinical practice guidelines recommend that watchful waiting be employed for three months in otherwise healthy children.6, x We volition search for relevant RCTs and observational studies that examined watchful waiting as a treatment strategy for OME in otherwise healthy children, special populations of children, and adults.
We have considered whether to include studies reporting outcomes by ears, rather than by subjects. Exclusion of studies by ears is reasonable and appropriate when: 1) the handling involved is systemic, or two) outcomes are measures of the patient's overall part, such every bit academic achievement, spoken language production, linguistic communication development, or quality of life. For ear-specific treatments or outcomes such as hearing thresholds or presence of fluid, ear-specific reports volition be included in this current review.
The Fundamental Questions
Proposed Key Questions (KQ)s were posted for public comment and the following concerns were expressed:
- Differences between KQs 1 and 2 are insufficient to justify ii questions.
- In KQ 5, health insurance coverage is not a wellness care–commitment characteristic, rather an enabler of health care commitment and therefore inappropriately included in a listing of health intendance–delivery characteristics.
- Geographic location and home environments were not included in the list of wellness care characteristics in KQ 5.
Later consulting with our Task Order Officer, we respond to each of these comments as follows:
- We volition continue KQs i and 2 as split up questions to conspicuously distinguish between clinical outcomes and function and quality-of-life outcomes. We believe that this is advisable because studies have shown that clinically measured effect levels may non parallel the patient's or parent'south perceptions of functionality. For case, the presence of middle ear fluid does non necessarily result in a perception of reduced hearing.
- In KQ five, to eliminate defoliation about the appropriate categorization of health insurance coverage, we have eliminated the phrase "health care-delivery characteristics." Outcome differences that can be attributed to health insurance are retained equally one of the factors that will be considered.
- In KQ five, we take antiseptic that location of the handling provider is more accurately described equally "type of facility of the treatment provider" and have added "geographic location" equally a factor that may touch treatment outcomes. We have not added "home environs" as this concept is vague and difficult to define in relation to its potential effect on treatment outcomes.
- In KQ5, we have replaced the give-and-take "modified" with "affected" to encompass the potential of the factors either modifying or mediating handling outcomes.
The revised and finalized KQs following public annotate are below. PICOTS were not affected past these changes in the KQs.
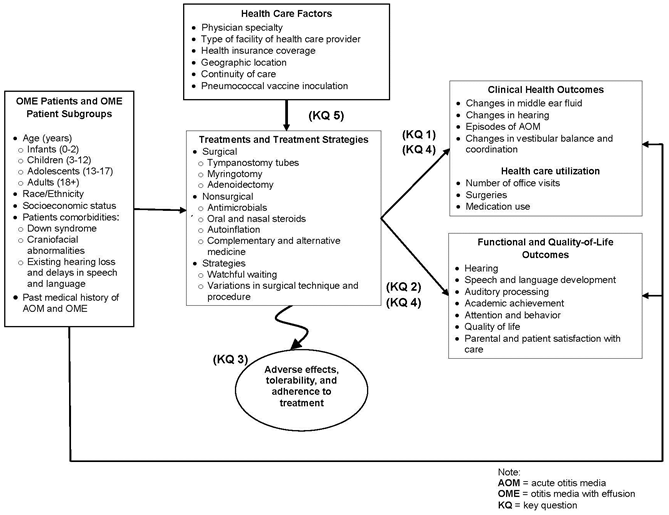
KQ 1: What is the comparative effectiveness of the following handling options (active treatments and watchful waiting) in affecting clinical outcomes or health care utilization in patients with OME? Clinical outcomes include changes in: OME signs (middle ear fluid) and symptoms (fullness in ear, difficulty in hearing), objective hearing thresholds, episodes of AOM, and vestibular function such as remainder and coordination. Treatment options include:
- Tympanostomy tubes
- Adenoidectomy with or without myringotomy
- Myringotomy
- Oral or topical nasal steroids
- Autoinflation
- Complementary and alternative medical procedures
- Watchful waiting
- Variations in surgical technique or procedure
KQ ii: What is the comparative effectiveness of the different treatment options listed in KQ 1 (active treatments and watchful waiting) in improving functional and health-related quality-of-life outcomes in patients with OME? Outcomes include: hearing, speech and linguistic communication development, auditory processing, academic achievement, attention and behavioral outcomes, wellness-related quality of life, and patient and parent satisfaction with intendance.
KQ 3: What are the differences in harms or tolerability amongst the unlike handling options?
KQ 4: What are the comparative benefits and harms of treatment options in subgroups of patients with OME? Subgroups include:
- Patients of different historic period groups
- Patients of different racial/ethnic backgrounds
- Patients in different socioeconomic status groups
- Patients with comorbidities such as craniofacial abnormalities (due east.g., cleft palate), Downwardly syndrome, and existing speech, language, and hearing bug
- Patients with a medical history of AOM or OME (with and without clinical hearing loss)
KQ v: Is the comparative effectiveness of treatment options affected past the following: health insurance coverage, md specialty, type of facility of the treatment provider, geographic location, continuity of care, or prior inoculation with the pneumococcal vaccine?
PICOTS Framework
| P: |
|
|---|---|
| I: |
|
| C: |
|
| O: |
|
| T: |
|
| S: |
|
Analytic Framework
Methods
A. Criteria for Inclusion/Exclusion of Studies in the Review
Studies will be included or excluded in the review based on the PICOTS model outlined in Section 2, findings from the topic refinement phase as described in section 1, and the report-specific inclusion criteria listed below in Table two.
| Category | Criteria for Inclusion |
|---|---|
| *Search to be updated when report is out for peer review. | |
| Study design | Meta-analyses, systematic reviews, RCTs, and nonrandomized controlled trials will be included for each treatment option. Prospective and retrospective accomplice studies and case-command studies volition be included for KQs that cannot exist answered using trial information alone due to gaps in the prove. |
| Study duration | Unlimited |
| Past ear or by subject studies | We will include both by ear and by discipline studies but will differentiate these two approaches when presenting results. |
| Time of publication | As described in section 1, some of the treatment options of interest accept been comprehensively addressed in recent Cochrane Collaboration or national regime-commissioned systematic reviews. For this reason and because of the large volume of literature on the topic, we volition search only for new literature when a handling has been adequately addressed in a review from 1 of these two types of sources. Each of the earlier reviews that come across this inclusion benchmark are listed below. We will include all studies identified by the relevant systematic reviews and volition synthesize the existing and new literature as one. An exception to this criterion will be cases in which our TEP finds the existing evidence compelling and the new evidence is unlikely to change earlier findings or probable to take a have a high risk of bias. In these cases, we volition simply comment on how the new literature adds to existing conclusions. We will search from 1948 forward for all treatments not addressed in one of the systematic reviews presented below. The following summarizes our search strategy for each included treatment choice and population of interest: The literature comprising nonrandomized and observational studies volition exist searched from 1948 frontward across treatment options. Tympanostomy tubes Adults: Special populations as outlined in our PICOTS : Adenoidectomy with or without myringotomy Adults: Special populations equally outlined in our PICOTS: Oral and topical nasal steroids Adults: Special populations as outlined in our PICOTS: Autoinflation Adults: Special populations as outlined in our PICOTS: Complementary and culling medical procedures Adults: Special populations as outlined in our PICOTS: Myringotomy Adults: Special populations as outlined in our PICOTS: Watchful waiting Adults: Special populations as outlined in our PICOTS: Variations in surgical technique or procedure Adults: Special populations every bit outlined in our PICOTS: |
| Language of publication | Given the volume of literature on this topic, nosotros will limit our search to publications in the English. |
B. Searching for the Evidence: Literature Search Strategies for Identification of Relevant Studies To Answer the Key Questions
We will systematically search, review, and analyze the scientific testify for each KQ. To identify articles for this review, we will carry focused searches of PubMed®, EMBASE®, the Cumulative Index of Nursing and Allied Wellness Literature (CINAHL®), and the Cochrane Library. An experienced research librarian will use a predefined list of search terms and medical subject headings (MeSH®) when applicable. Nosotros volition limit the search to studies published in English because of limited resources; this may bias the study to include more than studies from English language-speaking countries.
Equally described in Table 2 above, nosotros plan to vary the earliest publication date that we will search for RCT show based on the existence of studies having been previously identified in recent Cochrane Collaboration or national government-commissioned systematic reviews. We will, therefore, run multiple searches to obtain the almost focused meaningful show.
We will consummate targeted searches for unpublished or grayness literature relevant to the review. Methods for identifying gray literature will include a review of trial registries, specifically, ClinicalTrials.gov, Health Services Research Projects in Progress (http://www.nlm.nih.gov/hsrproj/), and the European Union Clinical Trials Register (https://www.clinicaltrialsregister.eu/). Farther, AHRQ will as well asking Scientific Information Packets from the developers or distributors of the interventions identified in the literature review. Scientific Information Packets allow an opportunity for the intervention developers and distributors to provide the Evidence-based Do Center (EPC) with both published and unpublished information that they believe should be considered for the review. The EPC volition review the information provided in the Scientific Data Packets and grey literature. We will include studies that meet all inclusion criteria and contain enough information on inquiry methods to be able to appraise the study's chance of bias.
We will likewise conduct an updated literature search (of the aforementioned databases searched initially) concurrent with the peer review process. Any literature suggested by peer reviewers or public comment respondents will be investigated and, if appropriate, incorporated into the final review. Reference lists of systematic reviews that are pertinent but do not meet our inclusion criteria will be scanned for studies that should be considered for this review. Appropriateness will be determined past the same inclusion and exclusion criteria described in the previous section.
C. Data Brainchild and Data Management
All titles and abstracts identified through searches will be independently reviewed for eligibility against our inclusion/exclusion criteria by two trained members of the research team. Studies marked for possible inclusion by either reviewer volition undergo a full-text review. For studies without adequate data to determine inclusion or exclusion, we will recall the full text and then make the determination. All results will exist tracked in an EndNote® (Thomson Reuters, New York, NY) database.
We will think and review the full text of all manufactures included during the title/abstract review phase. Each total-text article will be independently reviewed by 2 trained members of the research team for inclusion or exclusion on the basis of the eligibility criteria described earlier. If both reviewers hold that a study does not run into the eligibility criteria, the study will be excluded. If the reviewers disagree, conflicts will be resolved by discussion and consensus or past consulting a third member of the review team. All results will exist tracked in an EndNote database. Nosotros will record the reason why each excluded full-text publication did not satisfy the eligibility criteria so that nosotros tin can afterward compile a comprehensive listing of such studies.
For studies that meet the inclusion criteria, we will abstract relevant information into evidence tables. We will design information abstraction forms to assemble pertinent information from each article, including characteristics of study populations, settings, interventions, comparators, study designs, methods, and results. Trained reviewers will extract the relevant information from each included commodity into the bear witness tables. All data abstractions volition be reviewed for completeness and accurateness by a second fellow member of the squad.
D. Assessment of Methodological Quality of Individual Studies
To assess the risk of bias of studies, nosotros volition employ the guidance described in the Methods Guide for Effectiveness and Comparative Effectiveness Reviews.27 We will assess the potential for selection bias, performance bias, compunction bias, detection bias, and reporting bias. Results of this cess will exist summarized in a rating of depression, medium, or high risk of bias. In full general, a report with a low risk of bias has a stiff blueprint (more typically an RCT), measures outcomes appropriately, uses appropriate statistical and analytical methods, reports low attrition, and reports methods and outcomes clearly and precisely. Studies with a medium risk of bias are those that do non meet all criteria required for low risk of bias just do not have flaws that are likely to cause major bias. Missing information frequently leads to ratings of medium chance every bit opposed to depression hazard. Studies with a high risk of bias are those with at least ane major flaw that is likely to cause pregnant bias and thus might invalidate the results and includes errors in written report conduct or assay of results. Studies with a high risk of bias volition not be considered in this review. The questions included in the tools we will apply to evaluate hazard of bias will differ to some extent by study blazon (e.1000., RCT, nonrandomized trial, observational report) to examine the near disquisitional potential sources of bias that are likely to affect that design inside the context of this body of literature. Questions concerning the risk of bias of RCTs will be developed from the Cochrane Collaboration'due south tool for assessing risk of bias,28 and questions concerning the take chances of bias of nonrandomized and observational studies will be adult from the RTI Detail Bank on Run a risk of Bias and Precision of Observational Studies.27
Two independent reviewers will assess the risk of bias for each study. Disagreements between the two reviewers will exist resolved by discussion and consensus or by consulting a third member of the squad.
E. Data Synthesis
If we find three or more than similar studies for a comparison of interest, nosotros will consider quantitative analysis (i.e., meta-analysis) of the data from those studies. To determine whether quantitative analyses are appropriate, we will assess the clinical heterogeneity using the PICOTS framework and following established guidance.29 Nosotros will consider similarities and differences by PICOTS, sociodemographic factors (eastward.g., race, ethnicity, age, socioeconomic status), and study design. If quantitative analysis is appropriate, we will evaluate the statistical heterogeneity of pooled analysis using the chi-squared statistic and the I2 statistic (the proportion of variation in the study estimates due to heterogeneity).
When quantitative analyses are not appropriate (e.g., because of heterogeneity, insufficient numbers of similar studies, or insufficiency or variation in reporting), we will synthesize the data qualitatively.
F. Equivalence-Noninferiority
We will consider the potential equivalence (whether a new treatment is therapeutically similar to a standard treatment within a predefined margin of equivalence) and noninferiority—a newer treatment thought to be superior to an older handling on certain outcomes unrelated to effectiveness (e.g., fewer side effects, lower cost, and/or greater convenience) is not less constructive than the older treatment by some prespecified margin of acceptability. Whether we tin make these equivalence-noninferiority comparisons volition depend on whether a minimum important difference can be justified for item outcomes. Nosotros will make that determination early in the review process, before the evaluation of the included literature with input from our TEP.
G. Grading the Evidence for Each Key Question
We will course the overall force of the body of evidence on the basis of guidance established for the EPC Program.thirty This arroyo incorporates four fundamental domains: risk of bias (including written report blueprint and aggregate risk of bias across studies), consistency, directness, and precision of the evidence. The grades of show that can be assigned are described in Table 3. Grades reflect the strength of the torso of prove to answer the KQs on the comparative effectiveness, efficacy, and harms of the interventions in this review. Two reviewers will appraise each domain and the overall grade for each key outcome listed in the PICOTS framework, and conflicts will be resolved by consensus.
| Grade | Definition |
|---|---|
| Source: Owens et al., 201030 | |
| High | High conviction that the evidence reflects the true effect: Further research is very unlikely to modify our confidence in the estimate of effect. |
| Moderate | Moderate conviction that the show reflects the true effect: Farther research may change our confidence in the gauge of the upshot and may alter the estimate. |
| Depression | Low confidence that the testify reflects the true issue: Farther enquiry is likely to change our confidence in the guess of the consequence and is probable to alter the estimate. |
| Insufficient | Prove either is unavailable or does not permit estimation of an effect. |
H. Assessing Applicability
Nosotros volition assess the applicability both of individual studies and of the trunk of evidence. For individual studies, nosotros will examine weather that may limit applicability based on the PICOTS structure. Such conditions may be associated with heterogeneity of treatment issue and the power to generalize the effectiveness of an intervention to use in everyday practice.
To appraise the applicability of a body of prove, we will consider the consistency of results beyond studies that represent an array of different populations or a specific subpopulation of interest (e.g. individuals with comorbidities such every bit craniofacial abnormalities or Downwardly syndrome).
References
- Stool SE, Berg AO, Berman S, et al. Otitis Media With Effusion in Young Children. Clinical Practise Guideline No. 12. Rockville, Doc: Agency for Wellness Care Policy and Enquiry; 1994. AHCPR Publication No. 94-0622.
- Shekelle P, Takata G, Chan LS, et al. Diagnosis, History, and Tardily Effects of Otitis Media With Effusion. Evidence Report/Engineering Assessment No. 55 (Prepared by Southern California Show-based Do Center under Contract No 290-97-0001, Task Social club No. 4). Rockville, MD: Agency for Healthcare Research and Quality; 2003. AHRQ Publication No. 03-E023.
- Paradise JL, Rockette HE, Colborn DK, et al. Otitis media in 2253 Pittsburgh expanse infants: prevalence and risk factors during the get-go two years of life. Pediatrics. 1997 Mar; 99(3):318-33. PMID: 9041282.
- Tos Chiliad. Epidemiology and natural history of secretory otitis. Am J Otol. 1984 Oct; 5(6):459-62. PMID: 6542752.
- Williamson IG, Dunleavey J, Bain J, et al. The natural history of otitis media with effusion—a iii-year study of the incidence and prevalence of aberrant tympanograms in four South West Hampshire babe and first schools. J Laryngol Otol. 1994 Nov; 108(xi):930-4. PMID: 7829943.
- National Collaborating Centre for Women'due south and Children'due south Health. Surgical Management of Otitis Media With Effusion in Children. London: National Establish for Health and Clinical Excellence; Feb 2008. http://world wide web.prissy.org.great britain/nicemedia/alive/11928/39633/39633.pdf. Nice Clinical Guideline 60.
- Maw AR. Otitis media with effusion (glue ear). In: Kerr AG, Groves J, Evans NG, eds. Scott-Browan'due south otolaryngology. 5th ed. London: Butterworths; 1987:159-76.
- Finkelstein Y, Ophir D, Talmi YP, et al. Adult-onset otitis media with effusion. Arch Otolaryngol Caput Cervix Surg. 1994 May; 120(5):517-27. PMID: 8172703.
- Gravel JS, Wallace IF. Effects of otitis media with effusion on hearing in the start 3 years of life. J Speech Lang Hear Res. 2000 Jun; 43(three):631-44. PMID: 10877434.
- Rosenfeld RM, Culpepper 50, Doyle KJ, et al. Clinical practice guideline: Otitis media with effusion. Otolaryngol Head Neck Surg. 2004 May; 130(v Suppl):S95-118. PMID: 15138413.
- Sano S, Kamide Y, Schachern PA, et al. Micropathologic changes of pars tensa in children with otitis media with effusion. Arch Otolaryngol Head Neck Surg. 1994 Aug; 120(viii):815-19. PMID: 8049041.
- Madell JR. Impact of otitis media on auditory function. In: Rosenfeld RM, Bluestone CD, eds. Prove-based otitis media. Hamilton, Ontario, Canada: B.C. Decker Inc.; 1999:337-51.
- Hellstrom S, Groth A, Jorgensen F, et al. Ventilation tube handling: a systematic review of the literature. Otolaryngol Head Neck Surg. 2011 Sep; 145(3):383-95. PMID: 21632976.
- Williamson I. Otitis media with effusion in children. Clin Evid (Online). 2011 Jan 12; pii:0502. PMID: 21477396.
- Browning GG, Rovers MM, Williamson I, et al. Grommets (ventilation tubes) for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev. 2010; (10):CD001801. PMID: 20927726.
- van den Aardweg MT, Schilder AG, Herkert E, et al. Adenoidectomy for otitis media in children. Cochrane Database Syst Rev. 2010 Jan twenty; (1):CD007810. PMID: 20091650.
- Perera R, Haynes J, Glasziou P, et al. Autoinflation for hearing loss associated with otitis media with effusion. Cochrane Database Syst Rev. 2006 October xviii; (4):CD006285. PMID: 17054290.
- Thomas CL, Simpson S, Butler CC, et al. Oral or topical nasal steroids for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev. 2006 Jul 19; (3):CD001935. PMID: 16855980.
- Griffin GH, Flynn C, Bailey RE, et al. Antihistamines and/or decongestants for otitis media with effusion (OME) in children. Cochrane Database Syst Rev. 2006 Oct 18; (4):CD003423. PMID: 17054169.
- Williamson I, Benge Due south, Barton Southward, et al. Topical intranasal corticosteroids in 4-eleven year old children with persistent bilateral otitis media with effusion in chief care: double blind randomised placebo controlled trial. Clin Otolaryngol. 2010 Apr; 35(2):125-33.
- van Zon A, Schilder AGM, van der Heijden GJ. Antibiotics for otitis media with effusion in children (protocol). Cochrane Database of Systematic Reviews. 2011 June 15; (half dozen):CD009163.
- Rosenfeld RM, Bluestone CD, eds. Evidence-based otitis media. Hamilton, Ontario, Canada: B.C. Decker Inc; 1999.
- Which antibiotic for acute otitis media? Drug Ther Bull. 1974 Jul 5; 12(14):53-4. PMID: 4142433.
- Hassmann E, Skotnicka B, Baczek M, et al. Laser myringotomy in otitis media with effusion: long-term follow-upwards. Eur Curvation Otorhinolaryngol. 2004 Jul; 261(6):316-20. PMID: 14551787.
- Lin SH, Lai CC, Shiao As. CO2 laser myringotomy in children with otitis media with effusion. J Laryngol Otol. 2006 Mar; 120(3):188-92. PMID: 16359145.
- Sedlmaier B, Jivanjee A, Gutzler R, et al. Ventilation fourth dimension of the middle ear in otitis media with effusion (OME) after CO2 laser myringotomy. Laryngoscope. 2002 April; 112(4):661-viii. PMID: 12150520.
- Viswanathan M, Ansari MT, Berkman ND, et al. Assessing the risk of bias of individual studies when comparing medical interventions. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville, MD: Bureau for Healthcare Inquiry and Quality; March 2011. http://world wide web.effectivehealthcare.ahrq.gov/ehc/products/322/998/
MethodsGuideforCERs_Viswanathan_IndividualStudies.pdf. AHRQ Publication No. 12-EHC047-EF. - Higgins JPT, Alstman DG, Sterne JAC, eds. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. London: Chapter 8: Assessing gamble of bias in included studies. Available at: http://www.cochrane-handbook.org/; 2011.
- Fu R, Gartlehner G, Grant M, et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Programme. J Clin Epidemiol. 2011 Nov; 64(11):1187-97. PMID: 21477993.
- Owens DK, Lohr KN, Atkins D, et al. AHRQ series newspaper 5: grading the strength of a torso of bear witness when comparison medical interventions—Agency for Healthcare Research and Quality and the Effective Health-Intendance Plan. J Clin Epidemiol. 2010 May; 63(5):513-23. PMID: 19595577.
Definition of Terms
Astute otitis media: An acute infection of the center ear that tin can exist viral and/or bacterial in origin.
Audiometry: The testing of hearing power that includes determination of the hearing levels, ability to discriminate between various audio intensities, ability to distinguish speech from background noise and other aspects. Pure tone audiometry and impedance audiometry (tympanometry) are two of the ordinarily used tests for audiometric evaluation.
Autoinflation: A technique whereby the Eustachian tube (the tube that connects the middle ear and the back of the nose) is reopened by raising pressure in the nose. This can be accomplished past forced exhalation with closed mouth and nose, blowing up a balloon through each nostril or using an coldhearted mask. The aim is to introduce air into the eye ear, via the Eustachian tube, equalizing the pressures and allowing better drainage of the fluid.
Myringotomy: A surgical procedure in which an incision is fabricated in the tympanic membrane. It may be performed as a single procedure or as a grooming for insertion of a tympanostomy tube.
Otitis media with effusion: A drove of fluid in the middle ear without signs or symptoms of ear infection.
Otoscopy: The clinical examination of the ear canal and tympanic membrane, usually by ways of a hand-held auriscope (too known every bit an otoscope) providing illumination and magnification. Sometimes an attachment is used that permits insufflation of air into the ear culvert so that the mobility of the tympanic membrane can be assessed, and this is known as pneumatic otoscopy.
Tympanogram: A curve showing the manual of free energy through the middle ear at various air pressures in the external auditory canal. It gives a rough but objective cess of conductive hearing loss, and various middle ear disorders yield distinctive patterns of tympanogram:
- Tympanogram A: a symmetrical triangular graph with its peak at zero force per unit area level represents normal middle ear function.
- Tympanogram B: a apartment line on the graph represents the middle ear space filled with fluid, restricting movement of the tympanic membrane nether the externally applied pressure.
- Tympanogram C: this pattern is institute when there is a reduction of eye ear pressure level relative to the air force per unit area in the external auditory canal, which causes in retraction of the tympanic membrane; the graph shows the shift of the tympanographic superlative into the negative value range, but it is of a normal shape.
Tympanometry: Also known as impedance audiometry, the examination measures how readily the heart ear system (the tympanic membrane and the eye ear ossicles) can be ready into vibration with a change of air pressure in the external auditory canal. In the normal ear, maximum sound transmission occurs when the air pressure level within the middle ear space is the same as the atmospheric pressure, that is, equal to the air force per unit area in the external auditory canal.
Watchful waiting: Watchful waiting or active observation, equally it has more recently been called, is the process of regular review and followup of the kid, including assessments of hearing, development, and educational progress.
Summary of Protocol Amendments
| Date | Section | Original Protocol | Revised Protocol | Rationale |
|---|---|---|---|---|
| 7/xxx/2012 | II | In the Picots table; in the | In the Picots table; in "Placebo or no | To provide |
| 7/xxx/2012 | III | In the analytic framework, | Antimicrobials were | Antimicrobials |
| 7/thirty/2012 | Three | In the analytic framework, | Adherence is removed from the analytic framework. | Not pertinent to this Fundamental Question. |
Review of Cardinal Questions
For all EPC reviews, key questions were reviewed and refined as needed by the EPC with input from Central Informants and the Technical Expert Panel (TEP) to assure that the questions are specific and explicit well-nigh what information is being reviewed. In addition, for Comparative Effectiveness reviews, the key questions were posted for public annotate and finalized by the EPC after review of the comments.
Key Informants
Key Informants are the end-users of research, including patients and caregivers, practicing clinicians, relevant professional and consumer organizations, purchasers of health care, and others with experience in making wellness care decisions. Within the EPC program, the Cardinal Informant office is to provide input into identifying the Cardinal Questions for inquiry that volition inform health care decisions. The EPC solicits input from Key Informants when developing questions for systematic review or when identifying high-priority research gaps and needed new research. Fundamental Informants are non involved in analyzing the evidence or writing the study and have not reviewed the report, except every bit given the opportunity to do then through the peer or public review machinery.
Key Informants must disclose whatever fiscal conflicts of involvement greater than $10,000 and whatever other relevant business or professional person conflicts of interest. Because of their role equally end-users, individuals are invited to serve as Fundamental Informants and those who present with potential conflicts may be retained. The TOO and the EPC work to residue, manage, or mitigate any potential conflicts of involvement identified.
Technical Experts
Technical Experts incorporate a multidisciplinary group of clinical, content, and methodological experts who provide input in defining populations, interventions, comparisons, or outcomes as well every bit identifying detail studies or databases to search. They are selected to provide broad expertise and perspectives specific to the topic under development. Divergent and conflicted opinions are common and perceived as healthy scientific discourse that results in a thoughtful, relevant systematic review. Therefore study questions, blueprint and/or methodological approaches do not necessarily represent the views of individual technical and content experts. Technical Experts provide information to the EPC to place literature search strategies and recommend approaches to specific issues as requested past the EPC. Technical Experts practice not do analysis of any kind nor contribute to the writing of the report and take not reviewed the report, except as given the opportunity to do so through the public review mechanism.
Technical Experts must disembalm any financial conflicts of interest greater than $ten,000 and whatsoever other relevant business organisation or professional person conflicts of interest. Because of their unique clinical or content expertise, individuals are invited to serve equally Technical Experts and those who present with potential conflicts may be retained. The Too and the EPC work to residual, manage, or mitigate any potential conflicts of involvement identified.
Peer Reviewers
Peer reviewers are invited to provide written comments on the typhoon report based on their clinical, content, or methodological expertise. Peer review comments on the preliminary draft of the report are considered by the EPC in preparation of the terminal draft of the report. Peer reviewers do not participate in writing or editing of the last study or other products. The synthesis of the scientific literature presented in the final report does not necessarily represent the views of individual reviewers. The dispositions of the peer review comments are documented and will, for CERs and Technical briefs, be published 3 months after the publication of the Prove written report.
Potential Reviewers must disclose whatsoever financial conflicts of interest greater than $10,000 and any other relevant business or professional person conflicts of interest. Invited Peer Reviewers may not have whatever financial disharmonize of interest greater than $x,000. Peer reviewers who disclose potential business concern or professional conflicts of involvement may submit comments on draft reports through the public comment mechanism.
EPC Team Disclosures
None.
Role of the Funder
This project was funded under Contract No. HHSA 290-2007-10056-I from the Bureau for Healthcare Enquiry and Quality, U.S. Department of Health and Human being Services. The Task Order Officer reviewed contract deliverables for adherence to contract requirements and quality. The authors of this written report are responsible for its content. Statements in the report should not be construed as endorsement by the Bureau for Healthcare Enquiry and Quality or the U.S. Department of Health and Human Services.
Source: https://effectivehealthcare.ahrq.gov/products/ear-infection/research-protocol
0 Response to "what happens to the auditory ossicles in otitis media"
Post a Comment